Regulatory changes affecting pesticide registration during the 1980’s and 1990’s included Federal Good Laboratory Practices rules and the California Senate Bill 950.
Both resulted from a scandal involving fraudulent animal toxicology studies submitted in support of pesticide product registrations by the Industrial Bio-Test Laboratory in Northbrook, Illinois (NRDC 1983, NYT 1983, Qual Assourance, 2003). This resulted in a data-call-in to replace missing or inadequate studies for previously registered products. The requirement for new studies possibly factored in cancellation of many products during the 1980’s and 1990’s (ChE- last registration dates table). These outnumbered cancellations associated with individual public health events (e.g., phosalone 1987, 1988, 1990; mevinphos, 1993; parathion 1992)
Most new studies received detailed review and if relevant became subject to formal risk assessment process. This typically included identifying a no-effect level or no-adverse-effect-level from either animal or human studies and applying an appropriate safety factor . In contrast, the OSHA PEL’s and ACGIH TLV’s aimed at a either a no-effect-level or low-effect-level without an additional safety factor (AJIM 1990).
The 1996 Food Quality Protection ACT (FQPA) established regulation of pesticide AIs based upon MOAs common to chemical families and adding a requirement for an additional 10 fold safety factor for compounds sharing a common mechanism. This applied to exposures to residues in food, pesticides in drinking water, and from residential use.
Possibly because it may have seemed beyond the capability of the commonly available 1990’s bioresearch technology, FQPA did not establish a requirement for document MOA to register individual AIs.
It did create a category for reduced risk pesticides, based comparisons between alternative materials for particular use situations. For example, an evaluation in 2000 recognized buprofezin (see section below on compounds interfering with insect behavior, growth, and development) as having reduced risk compared to organophosphates for applications to cucurbit vegetables and head lettuce.
A 2002 evaluation granted reduced risk status for chlorfenapyr (see section below on disruptors of energy metabolism) in comparison organophosphates (especially chlorpyrifos) used for post construction termite control (US EPA, 2018).
Timeline of withdrawn or cancelled registrations
Arsenic, 1981
Copper acetoarsenite (Paris Green) Copper acetoarsenite (Paris Green) Last Ca registration inactive 01/20/81; Lacaris Green 962- 344-AA
DDT & other organochlorines
1972 DDT US EPA initiates phase-out in response to litigation by environmental advocacy group 1974
Chlordecone Neurologic illness at manufacturing operation, Hopewell Va, 76 (57%) of 133 former workers tested; secondary exposure to family members and community documented by blood testing
1983 Benzene hexachloride (BHC) Last Ca registration – BHC 2 DUST 7001-50386-AA – Inactive 01/17/83 1988
Toxaphene Last registered product – cattle ectoparasite treatment – Diptic 45639- 32-AA 1/1/1988 61% AI
Heptachlor Last registered product – Inactive 01/01/88 Gold Crest H-60 Insecticide Emulsifiable Concentrate 1990 Chlordane Last Ca registration date for chlordane formulation – Acme Chlordane 45% Spray 2217- 34-AA- 33955 1
1986 Dieldrin Last Ca registered product containing dieldrin inactive 1/4/1986 Aldrin Dieldrin 4-2 Granular 7001-50327-Aa; Dieldrin 1.5 EC 7001-50261-AA inactive 1/17/183
Aldrin last Ca registration 06/04/87 Niagara Aldrin 4.0 Miscible 279-50527-AA reported inactive 01/01/87
Endrin Last registered products – Endrin 1.6 EC 876- 153-AA inactive 01/01/87; Rid-A-Bird Control Liquid 7579- 1-AA inactive 01/17/90
Chlordecone Last Ca registration Dekko Silverfish Paks (trap/bait product) 3324- 5-AA INACTIVE 09/29/87 1997
Dienochlor Last Ca registration: Pentac Wp Miticide 55947- 95-AA inactive date 12/31/1997
Lindane Last Ca registration – Seed Mate Isotox Seed Treater (F) 66330- 19-AA- 36208 inactive 12/31/2005 2013 Dicofol Last Ca registration -Dicofol 4E 66222- 56-AA inactive 12/31/2013
Chlordimeform, Octopamine receptor analogues Last Ca registrations, inactive 1/1/87: Fundal 4 EC 45639- 75-AA,; Galecron 4E 100- 551-AA ; Galecron SP 100- 554-AA Fundal SP 45639- 73-AA – identification of chlordimeform as a cause of hemmorhagic cystitis in production workers and bladder cancer in animals, subsequent identification of cancer in cohort of European production workers
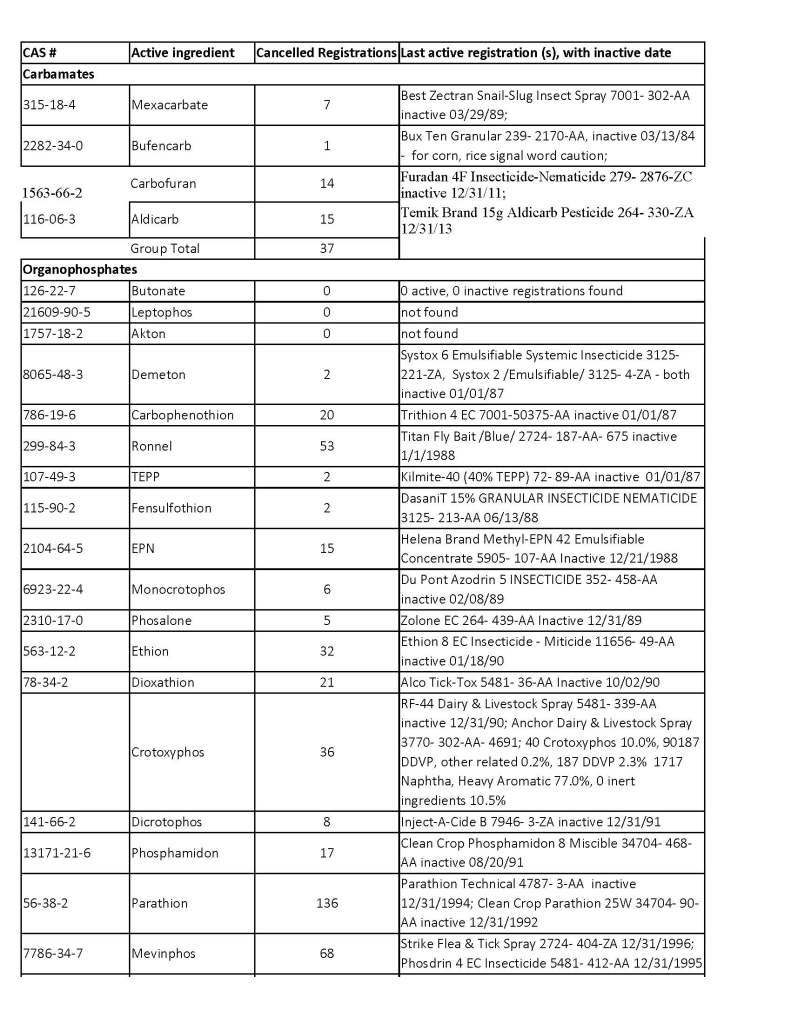
ChE inhibitors cancelled regulations

Leave a comment