Halide fumigants
Alkyl halogen fumigants ( ethylene dibromide, dibromochloropropane (DBCP), methyl bromide, dichloropropene, and chloropicrin) typify compounds with multi-site activity), each a reactive electrophile acting on multiple biochemical sites and on multiple organisms (insects, weed plants, and fungi).
2.3.3.1 -History
Roberts and Hutson (1999) described the mode of activity of halogenated hydrocarbaons as producing CNS depression, akin to a general anesthetic.
However, current understanding of anesthesia that CNS depression results from activation of a dual ion channel).
The traditional association of anesthetics with lipid solubility reflects the requirement for anesthetics to penetrate the blood brain barrier.
Most halide fumigants cause irritation of the skin, eyes, or respiratory tract (depending upon extent of contact). This may relate to a chemical mechanism also explaining their effect on weeds and fungi.
Carcinogenicity is recognized as an important secondary effect of many electrophiles (Miller and Miller, 1974). As discussed below, many of the alkyl halogens have required regulatory action because of carcinogenicity in animal tests. Electrophiles also have a propensity to cause both secondary irritant and allergic effects.
The sections below review toxicology, public health, and occupational health issues with individual compounds and chemical families.
History
Neifert described the insecticidal properties of halogenated fumigants in a report for USDA in 1924. Their sy/stematic application as fumigants began in the 1940’s, as indicated by a feature article in a Salinas, Ca newspaper in 1947, describing increased crop yields in experimental studies with ethylene dibromide and D-D mixture (DPR chemcode 185 1,2-dichloropropane, CAS # 78-87-5; PCID), 1,3-dichloropropene and related c3 compounds).
1974 vs 2016 fumigants
Halide fumigants used in 1974 and not used in 2016 included 1,2 dibromochloropropane (DBCP), ethylene dibromide, ethylene dichloride, and the D-D mixture (chemcode 185 1,2-dichloropropane (CID6564). Most have a high degree of reactivity and present safety issues because of their flammability, irritant properties (excerpts from pubchem warning information shown below), and toxic effects on insects, plants, fungi, nematodes, and vertebrates.
Dichloropropene and DD- mixture 1974 dichloropropene products found in 1974 accounted for 285 applications, including 284 with reported area treated, with an average application rate of 138 lbs per acre. 1 formulation (registration number 464-379) contained a mixture of 16.5% chloropicrin and 81.2% 1,3 dichloropropene. 14 products contained DD-mixture, accounting for an additional 739 applications. 3 of these contained chloropicrin, at concentrations between 15-57%. The average application rate equaled 109.8 lbs per acre.
2.3.3.2.1.1 dichloropropane – major health issue
A 1990 air monitoring survey by the California Department of Food and Agriculture found dichloropropene levels (177.8 µg/m3) far exceeding the risk assessment target (0.2 µg/m3) in Merced county, prompting suspension of use that lasted untilx. During this suspension a marked increased in the use of metam-sodium and the other MITC releasers. Newspaper press release copy citation
DBCP dibromo chloropropane
Following the introduction of DBCP in 1955, it had widespread use in the U.S.
DBCP does not cause the phytotoxicity associated with other alkyl halides, allowing post-plant crop treatment when desired for orchard and vineyard crops. It has moderate water solubility (Table 6), moderate acute toxicity and irritant properties, and a long environmental half-life (360 days).
Production grew from slightly more than 3,000,000 lbs in 1960 to 25,000,000 lbs in 1975 (Science total env 1981).
1974 California PUR data showed 30 products, registered to 13 different companies; Calculated summaries from electronic PUR data show 728,643.1013 lbs reported on 36,012.07 acres (average application rate of 26.33 lbs/acre). Treated commodities include multiple vegetable crops, orchard crops (citrus and nuts), cotton, ornamentals.
A publication in the Lancet (Whorton, 1977) described infertility in men who worked in a Lathrop Ca agricultural chemical formulating operation producing dibromochloropropane (DBCP) and other products. 11 men with 8 years employment in the operation had mean sperm counts of 0.2 X 106/ml, compared to 93 X 106/ml for 11 men with .08 years of employment. Evaluation of prior research on DBCB revealed a 1961 animal study showing similar effects:
The chemical suspected in the present investigation to be the cause of infertility had previously been shown to produce sterility in animals. D.B.C.P. was shown by Torkelson et al. to be toxic to the testes of rats, guineapigs, and rabbits. In the rat testis it caused degeneration of the seminiferous tubules, increase in Sertoli cells, reduced sperm-count, and abnormal sperm morphology. Rats with these effects also showed hepatic and renal degeneration. D.B.C.P. was found to produce these changes through skin absorption as well as ingestion or inhalation.
The absence of any notification to the workers exposed to DBCP prompted controversy (UCSF Synapse, 1980, California hearing notice in press Petaluma Argus Courier 1977, Selma Ca Enterprise 1981), as well as the development & promotion of risk communication programs as standard occupational health practice. Because of animal tests identifying as a carcinogen, DBCP well water contamination provoked concerns in at former sites of use in both California and Arizona. Tested sites of application in Texas proved negative. (Kingsburg Recorder 1977 – DBCP water contamination notification. Austin Statesman 1979 DBCP groundwater, none in Tx, postive Az, Ca).
Manufacture and exportation of DBCP abroad for years after its prohibition in the US provoked additional controversy. (IJOEH 1999, Lowry, Frank; Int J Health Services 2000).
2.3.3.3 Ethylene dibromide (EDB), CAS #106-93-4c
General Motors introduced tetraethyl lead (TEL) to improve gasoline combustion in the 1920’s, but found that an it required additional ingredient to prevented build-up of engine lead. Adding EDB allowing all of the lead to escape into the atmosphere. As noted above, USDA scientists described EDB’s insecticidal properties in 1925. Experimental studies in California during the 1940’s initiated its widespread use as a pre-plant soil treatment.
1974 use data showed 639 applications of EDB, a total 233,645.4 lbs of reported use. 488 application records contained information on treated area, a total of 11191.5 acres. The pre-plant application rate varied by commodity, averaging 29.2 lbs per acre. The most frequently treated individual commodities included carrots (33 applications, 1231 acres, at an average rate of 49.6 lbs/acre) and beans (93 applications, 9067 acres, 16.9 lbs/acre). The 16 products found in the 1974 data included a grain fumigant mixture (464-97, 1,2-Ethylene dichloride, 29 % Carbon tetrachloride 64% Ethylene dibromide 7%) and multiple products containing approximately 85% EDB.
Regulatory problems for EDB included a NIOSH animal study identifying the compound as a carcinogen in 1977. During the 1980’s EDB’s carcinogenicity became a limiting issue because residues in grain, prepared baked goods and baking mixes provoked broad public concern. Subsequent restrictions on EDB led to use of dichloropropene and other fumigant products.
The deaths of two workers cleaning an EDB contaminates storage tank in 1982 also caused public controversy (1982). The company physician disputed Cal-OSHA’s attribution of the deaths to EDB. A 1984 JAMA article reported elevated blood bromide levels in both employees (case 1 830 mg/L; case 2 380 mg/L compared to a reference level of < 4 mg/L). In a 1985 letter to JAMA Reesal, Shnitka, and Culver identified clostridial “pulmonary gas gangrene” as the cause of death in both employees, disputing the findings of the original JAMA report. Letz and his co-authors, in turn, emphatically disputed that interpretation of the case.
Methyl bromide 96-12-8
compound lies beyond the scope of this review. Appendix 1, on historical pesticide use, discusses information on the MOA for methyl bromide dating back to its trial use as a general anesthetic. A recent paper (Kim 2021) shows the possibilities of tranccriptomics and genomics in evaluating complex effects produced by fumigants on multiple biological processes and biochemical pathways.
In addition to the brief historical use of methyl bromide as an anesthetic (Appendix 1), it has had use as a refrigerant, a fire retardant because of its chemical stability and lack of flammability and as an intermediate in chemical manufacturing. Reported cases in the medical literature commonly resulted from accidents in chemical operations (Schuler, 1899; Jacquet 1901), mishandling of chemical fire extinguishers (), and fumigant use (Hine 1969, O’Malley 1997, Horowitz, 1997, O’Malley 2011, MMWR 2015).
Clinically, acute methyl bromide poisoning reflects its high degree of reactivity, causing damage to multiple organ systems, including respiratory, renal and hepatic failure, and severe neurological compromise (Benat, 1947, Araki, 1971, Horowitz 1998, O’Malley 1997, O’Malley 2011, Prathit et al 2015). Prolonged contact with the skin produce damage ranging from severe irritation to chemical burns (Zwaveling 1987; 1988). Methyl bromide is mutagenic in the bacterial Ames assay and positive in the sister chromatid exchange activity. Carcinogenicity tests demonstrate dose related squamous cell forestomach cancers in rats (Danse, 1984). This finding correlates with a dose related increase in the risk of stomach cancers in the agricultural health study (Barry et al 2012).
Despite the acute toxicity of methyl bromide, the most severe restrictions on its use arose from environmental concerns. The 1987 initial treaty to protect the ozone layer, amended in 1997, limited use of methyl bromide to essential crops, pending complete elimination in 2005 (United Nations Environment program, 1987). Nevertheless, after 2005, use continued under essential use exemptions for strawberries and other crops.
1974 data show 8 registered products for pre-plant fumigation and commodity fumigation in agriculture and for fumigation of structures. Of 6 labels found on the US EPA label search engine, 3 had legible information on application rates. The label for an agricultural product containing 50% methyl bromide and 50% chloropicrin (registration # 5316-9) recommended pre-plant use of 400-450 lbs per acre. For a product with 98% methyl bromide (registration # 5316-d31) the label recommended 200-600 lbs per acre. A product used for structural pest control (registration # 5316-41) carried a recommendation for use of 2.5-3.0 lbs per 1000 cubic feet.
2,135 application records for 1974 showed 600,096.5 lbs of reported use. For 126 records with treated area recorded, the average ,application rate equaled 336.6 lbs per acre.
Total use for 2016 amounted to 9,882,029.74 lbs sold, 2,602,822.51lbs reported use, with 728 applications to 11030.76953168 acres. For 686 pre-plant agricultural treatments, the application rate equaled 330 lbs per acre. It has minimal use as a structural fumigant, but still some use as a commodity fumigant.
The Montreal treaty prompted a search for alternatives to methyl bromide. These included propargyl bromide, eventually rejected because of its extreme reactivity, irritant and corrosive properties (Pubchem, 2021, MSDS Sigma Aldrich,), and the possible difficulties of handling it safely under field conditions. US EPA registered 5 methyl iodide products for a brief period between 2010 and 2012, but concerns about fetal thyroxicity and developmental toxicity prompted a very public controversy (Salinas, Californian 2011) that limited its use. 14 applications took place in 2011 and 2012, on cotton, outdoor nursey crops, grapes, treating 315.5 acres, a total of 1178.2 lbs. The registrant withdrew California registration for all 5 products in March 2012, possibly because of the limited market response.
Clinical toxicology
MeBr fatality
2009 – process leak in formulating operation – fatal acute respiratory compromise
| Patient complained of lungs burning. He also had superficial burns to his back and lower extremities. A few hours later he was intubated with: bp in the 60s, o2 sat around 70%, and temp of 92. He died later that day. | 45-ker-09. A worker in a methyl bromide formulation facility was exposed when a canister exploded. He was transported by ambulance to the hospital where he later died. (see 2009-697 and 2009-960) |
| Irritated eyes, headache, scratchy throat, mucous production. He was free of symptoms by the time of examination. | 45-ker-09. See 2009-696. The two ambulance workers who transported the exposed man were exposed when they opened a plastic bag containing the man’s wallet. They quickly developed symptoms. (see 2009-960) |
| Upon exposure, felt eye irritation and headache. At exam later that day, eyes felt “a little bit tired” and “kind of light sensitive”. | 45-ker-09. See 2009-696. They quickly ventilated the ambulance and their symptoms largely resolved. (see 2009-697) |
Methyl bromide off gassing in cold storage facilities
CFAM studies – offgassing in cold storage facility MMWR 2011 Illness Associated with Exposure to Methyl Bromide–Fumigated Produce — California, 2010
Please note: An erratum has been published for this article. To view the erratum, please click here.
Weekly
July 15, 2011 / 60(27);923-926
US Virgin Island s- https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6428a4.htm/
MITC releasing fumigants
The reactive compound methyl isothiocyanate (MITC) has a carbon-sulfur double bond, and a carbon-nitrogen double bond, leaving the carbon as an electrophile. MITC reacts readily with glutathione, amino groups and other nucleophiles.
Metam has marked irritant properties, initially noted as skin burns and severe irritation in handlers associated with pre-plant applications of metam for potato production in Germany (Wolf, Jung 1970, Jung 1975). Trials of aqueous mixtures metam with halide fumigants showed a high degree of incompatibility, attributable to the parent compound metam rather than its MITC byproduct (Pest Management Science 2005).
The section below reviews data on methyl isothiocyante (MITC) releasing fumigants, but also covering community exposure events for both metam and chloropicrin.
sensory irritation testing
Sensory irritation studies conducted on organic solvents typically demonstrate odor thresholds significantly lower than irritation thresholds (ACGIH). Reactive electrophiles, however, demonstrate a different pattern. Human volunteer studies of methyl isocyanate show eye irritation after 1-5 minutes, an immediately dangerous to life and health level of 3 ppm, and an odor threshold of 2-5 ppm. s [Mellon 1963].
https://www.cdc.gov/niosh/idlh/624839.html
An unpublished study conducted submitted to CDPR by metam manufacturers on human volunteers showed a
- Mean odor threshold 1,700 ppb volunteers
- 800 ppb mean threshold increased blink rate (1 hr exposure) and subjective irritation on ordinal scale
- 220 ppb NOEL
Although the irritation threshold reported in this study approximates the level for MIC, the much longer duration of exposure indicates that MIC has 10-50 fold greater irritant capacity than MITC.
Santa Barbara episodes
5-SB-92. Metam-sodium was shank-injected into a field and sealed in with a roller. No odor was noted afterwards. The air movement was stagnant later that evening. Northwest of the field, a family of 6 developed symptoms. See 92-166 to 170.
25-SB-99. The odor from various metam-sodium applications affected 2 business owners and their children while at the shop. Two days later, similar odors affected children and staff at a nearby elementary school. See 1999-1169 to 1175 total 7 case.
When an employee began applying a pesticide inside sewage pipes, he felt he inhaled the fumes and stopped the application. He wore protective equipment, but developed symptoms anyway. He sought medical attention the next day. La 1998-1353
2002 & 2004 LA formulating operation
2002-1440
An inexperienced operator splashed metam-sodium on his forearms in the course of changing filter bags on the production line. He consulted a doctor 11 days later. The doctor directed him to avoid chemical exposure until he recovered. Itchy rash on both forearms.
2004-122
Itchy, painful and swollen rash on the arms, hands and face.
A chemical assistant operator attributed a rash to fume contact while transferring metam-sodium into a drum. He told his supervisor six days later, and was sent for care. He worked light duty for two and a half months while the doctor monitored progress.

LA DWP 1982 – 3 LA DWP employees – ? 1982 – illness report – or 1982-1990 data
Dichobenil , metam mixure1998-1353
When an employee began applying a pesticide inside sewage pipes, he felt he inhaled the fumes and stopped the application. He wore protective equipment, but developed symptoms anyway. He sought headache, burning in both eyes, numbness all over the body, nausea, vomiting. Medical attention the next day
2002-1440
Episodes following restrictions on dichloropropene
San Joaquin11 5 1993
San Joaquin – 9 26 1995 – 14 manufacturing workers – offgassing from adjacent appoication
10/16/1996 28 students 1 teacher – Juveinile authority facility adjacent
Tulare Co – Earlimart – 11 13 1999

25,000 pounds metam-sodium applied adjacent to isolated area NE of Arvin, July 8, 2002 252 cases eye and upper respiratory irritation, non-specific systemic symptoms, and lower respiratory complaints Michael O’Malley, MD, MPH Terrell Barry, PhD Mario Ibarra, BS Marylou Verder-Carlos, DVM, MPVM Louise Mehler, MD, PhD Journal of Agromedicine, Vol. 10(4) 2005 Illnesses Related to Shank Application of Metam-Sodium, Arvin, California, July 2002
Chloropicrin trichloronitromethane CID: 6423 CAS # 76-06-2 MF: CCl3NO2
MW: 164.37g/mol

Chloropicrin has demonstrated irritant properties since its use as chemical weapon in World War 1. Its use as a soil fumigant gas has allowed lower concentrations of dichloropropene and methyl bromide in numerous formulations.
As demonstrated in a study of human volunteers, it causes irritant symptoms at concentrations between 50 & 100 ppb (O’Malley, J Agromedicine 2005). RECT chloropicrin
Lamont episode MMWR

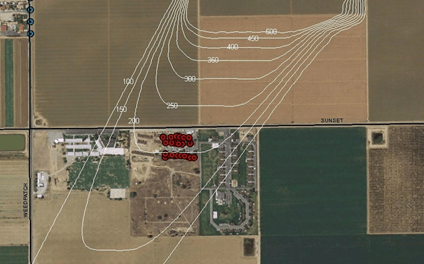
Salinas 2005
J Agromedicine 2007
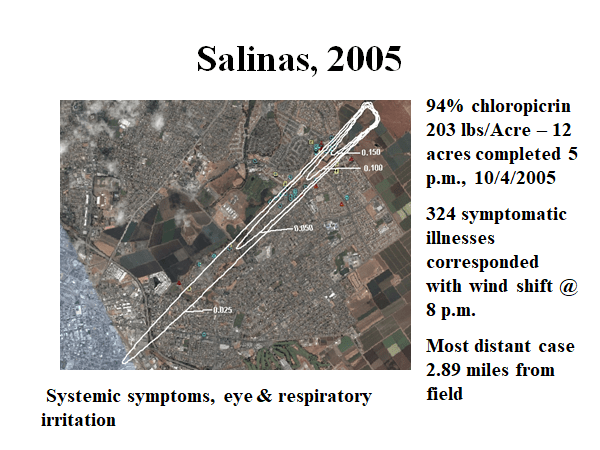
2005 Salinas chloropicrin episode J Agromedicine, 2007 stagnant night time condition – 94% chloropicrin 203 lbs/Acre – 12 acres completed 5 p.m., 10/4/2005 324 symptomatic illnesses corresponded with wind shift @ 8 p.m., Most distant case 2.89 miles from field

Leave a comment