IRC 19 Octopamine receptor /agonists – 1974 – Chlordimeform; 2016 -Amitaz
Octopamine receptor agonists (IRC 19):
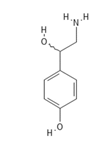
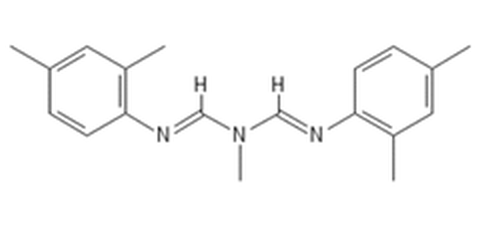

Octopamine functions as an adrenergic transmitter in insects and other invertebrates but also has stimulant properties in mammals. In 1974, the octopamine receptor agonist chlordimeform (chemcodes 300, 301) had 316,157.2 lbs of reported use (0.55% of total). Applications on cotton on cotton accounted for 89% of chlordimeform use, with minor uses on orchard and vegetable crops. Despite its comparatively limited use, it became a focus of concern because of its secondary effects.
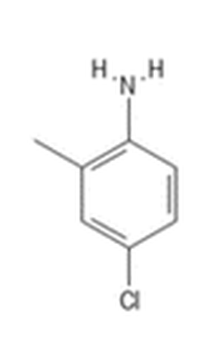
Use of chlordimeform became very tightly controlled because of an episode of hemorrhagic cystitis in a chlordimeform packing operation in 1975 (Kimbrough, JAMA, 1976) and an animal study showing a high incidence of hemangiosarcoma. Bladder cancer was observed (1987) in German workers handling chlordimeform’s principal metabolite 4-chloro-ortho-toluidine (4-COT). It was last registered in California in 1987 (Table 3b). As discussed below, the only octopamine receptor compound currently registered is amitraz. The octopamine analogue compound amitraz has veterinary use in control of ectoparasites; as a pesticide it is used to control varoa mites in bee hives. 2016 California data showed 4 of 35 historical registrations for amitraz, including a formulation for treating Varoa mites in apiaries, and 3 formulations for pet ectoparasites. Prior registrations included specialty use on single crops: cotton, pears, and citrus.
2016 data showed 1639 lbs sold, 0.0008% of total; 27.6 lbs reported agricultural use, 0.000020 % of total; 27 applications 0.0026 % of total. All reported use involved treatments of beehives for Varoa mites.
Toxicity data
Amitraz has moderate mammalian ` on oral administration but shows markedly less toxicity on skin contact (Table 5 – octopamine receptor agonist amitraz, IRC-19 and illustrations of related chemical structures). The 2016 data show 703 lbs of California sales for amitraz, but limited agricultural use. California data shows initial registration in the 1980’s as a treatment for mite infestations on pears (11/27/1984, initial registration date not listed). Peak use occurred between 1994 and 1997, averaging 66,862 lbs/year (for control of mites on pears, citrus and cotton).
The use reporting and sales data do not track prescription veterinary medications (e.g., Mitiban, used for control mange), but do record treatments for pig, cattle and dairy ectoparasites marketed directly to ranch or farm operators. 3 current registrations include a treatment for apiaries infected with Varoa mites and 2 tick collars for dogs.
The amitraz toxidrome depends upon the amount of exposure, with alpha-adrenergic symptoms and reflex cholinergic at high doses producing respiratory depression, miosis and bradycardia suggestive of contact with a ChE-inhibitor. California illness data show 2 cases with amitraz as the sole implicated pesticide (Table 3b), 1 case of accidental direct eye exposure (1992) and 1 accidental ingestion (2018).
A 2011 case reported from Iran described a 22 years old university student who intentionally ingested about 100 cc of an amitraz formulation 3.5 hours before presenting to the hospital with nausea, vomiting, and dizziness and constricted pupils, one adult dose of activated charcoal (50 g) and referred her to our Poisoning Emergency Department, where she was managed supportively and successfully.
A 2006 case series from Turkey described 23 adult patients (16-78 years) affected by ingestion (7 intentional 11 accidental), inhalation or skin contact with amitraz (clinical toxicology 2006). Prominent symptoms in the ingestion cases (resembling California case 2018-512, described above) included vomiting, altered consciousness, and drowsiness. Because of bradycardia or miosis, providers initially diagnosed ChE-inhibitor poisoning in 9 patients, 7 of whom received treatment with ,
Pediatric poisoning typically involve accidental ingestion of liquid formulations available in the home, illustrated by cases reported from rural Argentina (2009, bradycardia, hypotension in a 2 year old), and India (2013, intentional ingestion in a 13 year old presenting with hypotension, coma and respiratory failure, initially suspected to
2016 data showed 1639 lbs sold, 0.0008% of total; 27.6 lbs reported agricultural use, 0.000020 % of total; 27 applications 0.0026 % of total. All reported use involved treatments of beehives for Varoa mites.
Toxicity data
Amitraz has moderate mammalian ` on oral administration but shows markedly less toxicity on skin contact (Table 5 – octopamine receptor agonist amitraz, IRC-19 and illustrations of related chemical structures). The 2016 data show 703 lbs of California sales for amitraz, but limited agricultural use. California data shows initial registration in the 1980’s as a treatment for mite infestations on pears (11/27/1984, initial registration date not listed). Peak use occurred between 1994 and 1997, averaging 66,862 lbs/year (for control of mites on pears, citrus and cotton).
The use reporting and sales data do not track prescription veterinary medications (e.g., Mitiban, used for control mange), but do record treatments for pig, cattle and dairy ectoparasites marketed directly to ranch or farm operators. 3 current registrations include a treatment for apiaries infected with Varoa mites and 2 tick collars for dogs.
The amitraz toxidrome depends upon the amount of exposure, with alpha-adrenergic symptoms and reflex cholinergic at high doses producing respiratory depression, miosis and bradycardia suggestive of contact with a ChE-inhibitor. California illness data show 2 cases with amitraz as the sole implicated pesticide (Table 3b), 1 case of accidental direct eye exposure (1992) and 1 accidental ingestion (2018).
A 2011 case reported from Iran described a 22 years old university student who intentionally ingested about 100 cc of an amitraz formulation 3.5 hours before presenting to the hospital with nausea, vomiting, and dizziness and constricted pupils, one adult dose of activated charcoal (50 g) and referred her to our Poisoning Emergency Department, where she was managed supportively and successfully.
A 2006 case series from Turkey described 23 adult patients (16-78 years) affected by ingestion (7 intentional 11 accidental), inhalation or skin contact with amitraz (clinical toxicology 2006). Prominent symptoms in the ingestion cases (resembling California case 2018-512, described above) included vomiting, altered consciousness, and drowsiness. Because of bradycardia or miosis, providers initially diagnosed ChE-inhibitor poisoning in 9 patients, 7 of whom received treatment with ,
Pediatric poisoning typically involve accidental ingestion of liquid formulations available in the home, illustrated by cases reported from rural Argentina (2009, bradycardia, hypotension in a 2 year old), and India (2013, intentional ingestion in a 13 year old presenting with hypotension, coma and respiratory failure, initially suspected to be OP poisoning), and Turkey (series of 11 cases, 2000).
be OP poisoning), and Turkey (series of 11 cases, 2000).
Leave a comment